Abstract
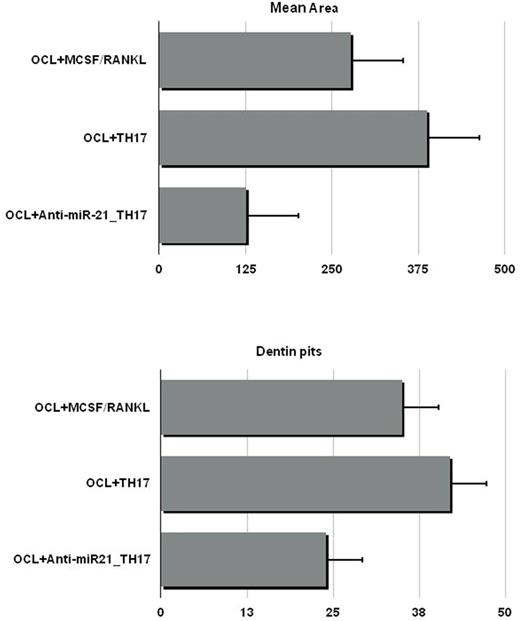
Bone disease (BD) represents a hallmark of multiple myeloma (MM), characterized by classical osteolytic lesions and/or osteoporosis, that can be life threatening and contribute to poor outcomes. The complex interplay among MM conditioned bone effectors such as MM cells, osteoclasts (OCLs) and osteoblasts within BM microenvironment (BMM) supports MMBD. Several studies have shown that the extent of BD is tightly correlated to Th17 cells development and maintenance. Th17 secrete pro OCL cytokines such as RANKL, TNF, and IL-17, contributing to MMBD development. Recent findings showed that microRNAs (miRNAs) are involved in MMBD. MiRNAs are short non-coding RNAs that regulate gene expression at a post transcriptional level. Among others, miR-21 is involved in Th17 differentiation by targeting SMAD-7, a negative regulator of TGF-β signaling, promoting the activation of SMAD-2/3, suppressing IL-2 expression, and ultimately enhancing the activity of the TGF-β signaling pathway. Taken together, these data support a therapeutic strategy of miR-21 targeting to ameliorate MMBD through Th17 inhibition. We have previously demonstrated that miR-21 activity within BMM plays a crucial role in bone resorption/apposition balance through PIAS3 suppression and STAT3 dependent RANKL transcription in BM stromal cells. In the present study, we sought to evaluate the role of miR-21 in Th17 mediated BD. We enriched Th17 from BM CD4+CCR6+ T cells and evaluated miR-21 levels. We found increased miR-21 in MM derived Th17 (MMTh17) as compared to controls, supporting the relevance of miR-21 in MMTh17. To further explore the mechanisms of miR-21 activity in Th17 function, freshly isolated naïve CD4+ T cells were differentiated from healthy donors into Th17 along 18 days of culture in the presence of polarizing cytokines. At the end of culture, T cells expressed the Th17 full maturation markers RORC and IL17. As a model of MMBD in vitro, OCL resorptive activity was evaluated on dentine discs with/without Th17 freshly isolated from MM patients or differentiated in vitro. In both cases, Th17 promoted potent OCL mediated dentin pits generation as compared to controls. To counteract miR-21 activity, we electroporated naïve CD4+ T cells with miR-21 inhibitor (miR21i) or scramble control mirna before starting Th17 differentiation culture. MiR-21 expression levels were assessed by rt pcr before and at the end of differentiation culture to ascertain its effective inhibition. To map signaling pathways differences elicited by miR21i-Th17, we performed a mass spectrometry-based quantitative phosphoproteomics analysis. 1134 phosphorylated proteins were identified, with 386 significant changes as compared to controls. The global-pairwise-normalized data underwent to Ingenuity Pathway Analysis (IPA). Interestingly, analysis showed a generally higher level of phosphoserine sites and top canonical pathways affected by miR-21 inhibition included PI3K/AKT and 14-3-3 Signaling. Among Th17 potential regulators, enhanced activity of PDCD4, STAT1, MINK1 and FOXO1 were observed, while ITGAL showed reduced activity. On these bases, we performed WB to validate IPA analysis and explored additional widely acknowledged Th17 regulators. Upon miR-21i treatment, the expression of PDCD4 and FOXO1 were significantly increased while lower levels of RORC were observed. Furthermore, PIAS3 and TBX21 were significantly upregulated in the presence of miR-21 antagonism. STAT3 is a master transcription factor for Th17 upon IL-6 stimulation. As PIAS3 and PDCA4 negatively regulate STAT3, we checked phospho-STAT3 expression and found it downregulated as expected. Overall, these data indicated that Th17 differentiation was impaired by miR-21i. In the second set of experiments, we aimed to evaluate Th17 functional impairment by miR-21 antagonism in MMBD. OCLs were plated with dentine discs and exposed to either Th17 alone, miR21i-Th17, or RANKL/MCSF cytokine alone as positive control. Dentine pits and areas were significantly lower in the presence of miR21i-Th17 as compared to Th17 alone and positive control (Fig.1). These data indicated that miR-21 antagonism negatively regulates Th17 mediated OCL activity and support the role of miR-21 inhibition as an innovative treatment tool in MMBD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal